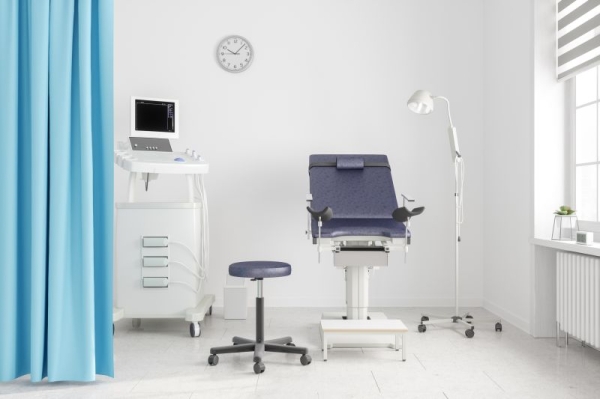
Instead of a traditional speculum-involved pelvic exam to screen for cervical cancer, the US Food and Drug Administration has given the go-ahead for patients to have the option to collect their own vaginal samples for screening in a health care setting, such as at their doctor’s office, an urgent care or even a mobile clinic.
Two health care businesses – biotechnology company Roche and medical technology firm Becton, Dickson and Company or BD – announced Wednesday that the FDA approved the use of self-collected samples with their respective HPV tests.
Most cervical cancers are caused by human papillomavirus or HPV, and screening for HPV can help identify women who may be at risk of developing cervical cancer.
“Almost all cervical cancers are caused by persistent infection with certain types of HPV,” Dr. Karen E. Knudsen, CEO of the American Cancer Society, said in a statement Wednesday. “Self-collection can expand access to screening and reduces barriers, which will give more people the opportunity to detect, treat, and ultimately survive cancer.”
A pediatrician gives an HPV vaccination to a 13-year-old girl. Getty
Typically, gynecologists collect samples for HPV testing, a cervical cytology or both. Cervical cytology, also known as a Pap test or Pap smear, involves examining cervical cells for changes to find precancerous or cancerous cells.
The US Preventive Services Task Force recommends screening for cervical cancer with cervical cytology every three years for women ages 21 to 29. For women ages 30 to 65, the USPSTF recommends screening every three years with cervical cytology alone, every five years with high-risk HPV testing alone, or every five years with high-risk HPV testing in combination with cytology.
But for some patients, having a provider collect a sample for those tests can be painful or awkward.
“Many patients are uncomfortable with the intimate nature of a pelvic exam,” Dr. Jeff Andrews, a board-certified gynecologist and vice president of global medical affairs for diagnostic solutions at BD, said in the company’s announcement Wednesday.
“Also, many people live in areas without a local doctor or clinician trained to obtain a sample with a speculum,” he said. “The option to self-collect in a clinical setting can help women overcome some of these barriers.”
Doctor examing woman`s stomach with stethoscope – stock photo Joos Mind/The Image Bank RF/Getty Images
The BD Onclarity HPV Assay is now FDA-approved for HPV testing on self-collected samples without the need for a traditional Pap test, according to the company’s announcement Wednesday. A trial on self-collection for HPV testing, which BD is participating in with the National Cancer Institute, is expected to begin enrolling this summer, the company said, to evaluate accuracy of self-collection for HPV testing in health care and other settings, including at home.
Roche announced that HPV self-collection is approved for use with the company’s cobas HPV test, and the company has also collaborated with the National Cancer Institute.
Each year in the United States, more than 11,000 cases of cervical cancer are diagnosed and about 4,000 women die of the disease, according to the US Centers for Disease Control and Prevention. It’s estimated that about half of invasive cervical cancer cases are diagnosed in people who have never been screened and about 10% of diagnoses are in people who have not had a Pap test in the five years prior.
“Despite the benefits of cervical cancer screening, not all women and people with a cervix get screened regularly,” Dr. William Dahut, chief scientific officer at the American Cancer Society, said in a statement Wednesday.
“Most cervical cancers are found in people who have never had a cervical cancer screening test or who have not had one recently. That’s why adding self-collection in a health-care center as a screening method for this potentially deadly disease can make a huge impact,” he added. “We anticipate self-collection in a health-care setting will play an increasingly prominent role in cervical cancer screening once regulatory and clinical prerequisites are in place and as supporting evidence continues to accumulate.”
Get CNN Health’s weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
Next, the FDA could consider greenlighting the self-collection method at home instead of just in a health care setting. Teal Health has developed a home cervical cancer screening device called the Teal Wand that was granted “breakthrough device” status this month by the FDA, which would allow the agency to review the device on a faster timeline.
“FDA’s recognition of the Teal Wand as a Breakthrough device acknowledges the important public health benefit that self-collection for cervical cancer screening can have on those who are rarely screened or who do not participate in clinician-based screening for cervical cancer,” Trena Depel, vice president of clinical and regulatory at Teal Health, said in a news release at the time.
Screening for cervical cancer remains important because early cases often may not have signs or symptoms. Advanced cases may cause abnormal vaginal bleeding or unusual discharge. Cervical cancer is treated in many ways, including surgery, chemotherapy and radiation therapy.
Some of the most important steps women can take to help prevent cervical cancer, according to the CDC, are to get vaccinated against HPV, not smoke, use condoms during sex, have regular screening tests and go back to see the doctor if their screening test results are not normal.

